High-Quality Mass Culture: Cell Processing Center with Reliable Quality Assurance
Cell Processing Center and Reliable Quality Assurance Supporting High Quality × Mass Cultivation
Our exosome supernatant “High Concentration Factor 9” undergoes
strict quality testing of its source cells.
Additionally, all batches of “High Concentration Factor 9” undergo complete inspection before shipping.
We deliver safe and reliable products.
Testing at Cell Reception
- ・Cleared safety tests in accordance with the Ministry of Health, Labour and Welfare’s Biological Raw Material Standards (Established February 28, 2018, MHLW Notice No. 37)
- ・Sterility test
- ・Endotoxin test / Mycoplasma test
- ・Virus negative test (HBs antigen/antibody, HBc antibody, HTLV-I antibody(CLIA), HIV antigen/antibody, Syphilis(PRP/TPHA))
- ・Endotoxin test / Mycoplasma test
- ・Inspection at supernatant shipping
- ・Sterility test
- ・Endotoxin test / Mycoplasma test
- ・Cell-free diagnostic test

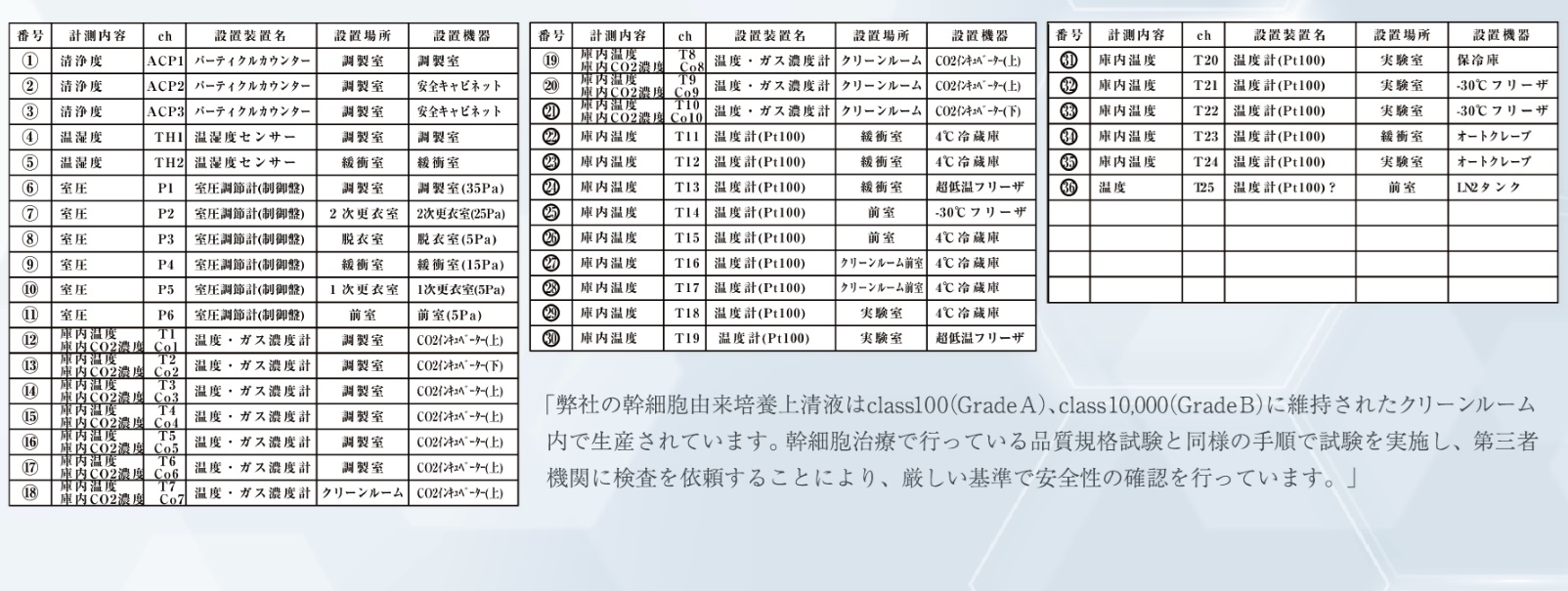
CPF Clean Room Facility Specifications (GMP Compliant)
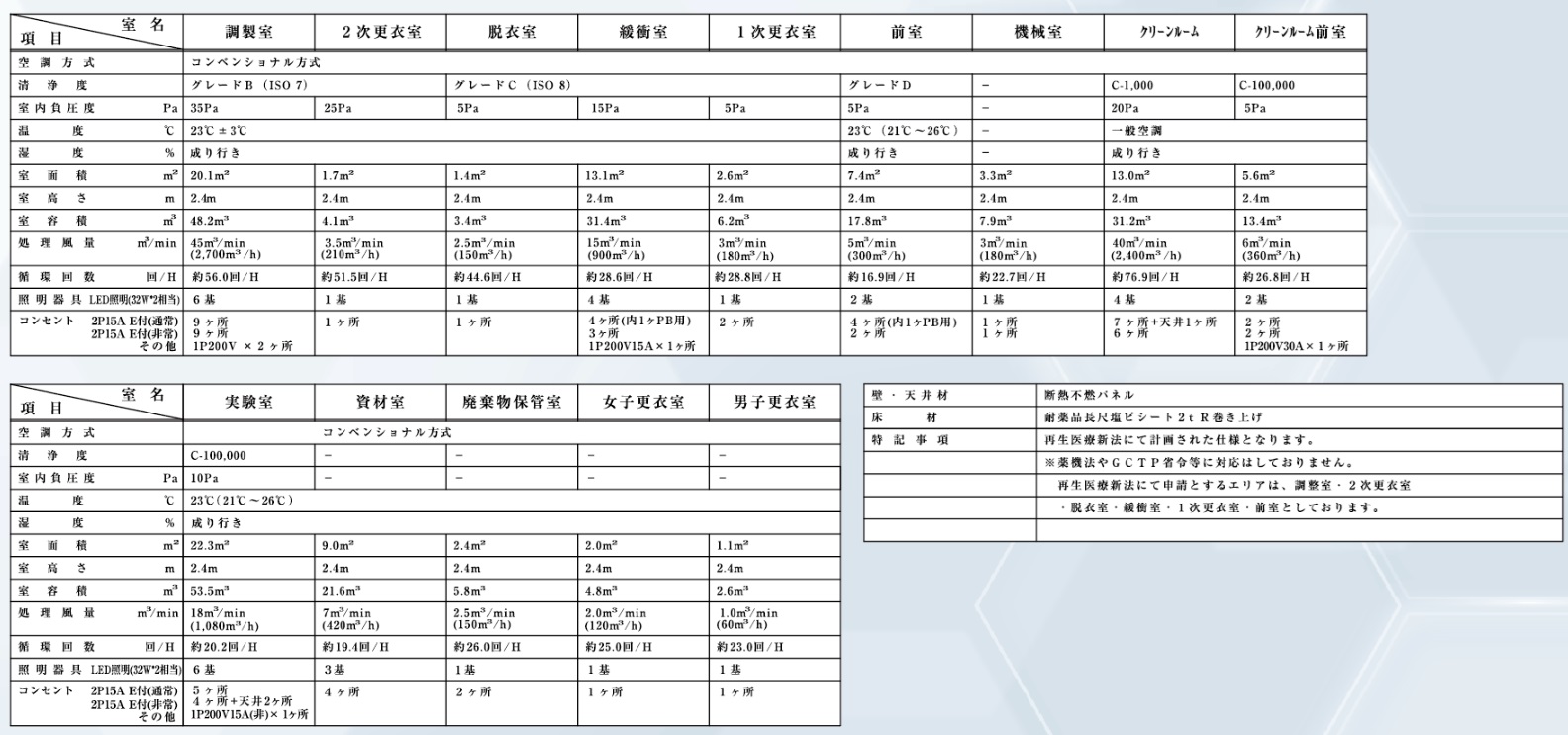
CPF Clean Room Facility Specifications (GMP Compliant)